The Isolated
Blood And Perfusion fluid Perfused Heart
Fiona J Sutherland and
David J Hearse
Cardiovascular Research
The Centre for Cardiovascular Biology and Medicine
The Rayne Institute, King’s College
St Thomas’ Hospital, London SE1 7EH, United Kingdom
Email: david.hearse@kcl.ac.uk
Telephone: 44 0207 928 9292 ext. 5711, Fax: 44 0207 922 8139
The isolated heart as a model
for the study of cardiovascular disease.
As discussed in an earlier paper1, the
isolated perfused small mammalian heart probably represents the
optimal compromise in the conflict between the quantity and
quality of data that can be acquired from an experimental model
versus its clinical relevance - especially in relation to the
modelling of ischemia. In exploiting this preparation to its full
potential it is important to consider a number of key questions
each of which will be addressed in the following sections. In the
course of this discussion, a practical guide to perfusion
methodology will emerge.
What are the advantages and
disadvantages of the isolated perfused heart?
At a practical level, the isolated heart,
especially from small mammals, provides a highly reproducible
preparation which can be studied quickly and in large numbers at
relatively low cost. It allows a broad spectrum of biochemical,
physiological, morphological and pharmacological indices to be
measured (see later). These measurements can be made in the
absence of the confounding effects of other organs, the systemic
circulation and a host of peripheral complications such as
circulating neurohormonal factors. This characteristic may be
considered as an investigational advantage in that it allows the
dissection of peripheral from cardiac responses or a disadvantage
in that it makes the preparation one step further removed from
the in vivo state. Whilst the fact that the isolated heart is
denervated must always be taken into account, this can be turned
to advantage allowing the separation of cardiac from sympathetic
and vagal stimulation. Denervation and the absence of other
peripheral factors can often be compensated for; thus,
catecholamines or other neurotransmitters may be included in
perfusates and many other peripheral factors can be added
exogenously and in a controlled manner which again can represent
a significant investigational strength. Certainly, the isolated
perfused heart provides an excellent test-bed for undertaking
carefully controlled dose-response studies of metabolic or
pharmacological interventions.
It must be recognised that, as an ex vivo
preparation, the isolated heart is a constantly deteriorating
preparation but nonetheless it is capable of study for several
hours. The preparation also readily allows the induction of whole
heart or regional ischemia and this can be achieved at various
levels of flow. Similarly, anoxia or hypoxia at various degrees
of oxygen deprivation (in the presence of normal flow) can be
easily imposed. The isolated heart preparation is amenable to
reperfusion or reoxygenation at various rates and with various
reperfusate compositions thus providing a powerful tool for
assessing many aspects of ischemia- and reperfusion-induced
injury. Arrhythmias are readily induced and studied, especially
in the larger hearts where conduction pathways can be mapped and
a variety of electrophysiological recordings made. Of particular
importance, the isolated heart preparation allows experiments to
be continued in the face of events (e.g. infarction-induced loss
of pump function, cardiac arrest or arrhythmias) which would
normally jeopardise the survival of an in vivo experiment.
Which species is best for
perfusion?
In essence the hearts from any mammalian
species (together with non-mammalian hearts such as those from
frogs or birds) may be perfused. However, although isolated
perfusion of large animal hearts such as pigs, monkeys, sheep,
dogs and even man has been reported, these are less frequently
used. This is probably on account of the high cost, greater
variability, large volumes of perfusion fluids and cumbersome
equipment that is required. Without doubt the most frequently
studied heart is that of the rat but there are numerous reports
of studies with other species such as the rabbit, guinea pig,
hamster, gerbil, ferret and mouse.2 The advent of
transgenic technology will undoubtedly result in increasing
numbers of studies using murine preparations and this will
require investigators to become adept at using this, as yet,
poorly characterised model. Unfortunately, although the
literature contains an increasing number of studies which employ
mouse hearts, the fundamental characteristics of the preparation
(e.g. pressure-volume and calcium-function relationships) have
yet to be completely characterised. Another caution relates to
the high heart rate of the mouse and the miniaturisation of
equipment which requires investigators to take due account of the
frequency-response limitations of recording equipment.
In practical terms, the rat heart is by
far, the best characterised, it is also the heart most frequently
used for more complex perfusion preparations such as working and
blood perfused hearts. In terms of ease of handling, the rat has
a great advantage over smaller hearts such as the mouse where
intraventricular pressure recordings are more difficult. The rat
does however suffer from one, frequently cited, limitation,
namely its very short action potential duration which can limit
its value (in terms of extrapolating to the human) of some
studies of arrhythmogenesis and anti-arrhythmic drugs. Other
species, such as the rabbit, suffer problems with anesthesia and
the guinea pig heart differs from other species in that it is
totally collateralised effectively preventing the study of
regional ischemia in this species. Thus, as no ideal species
exists, in selecting a species for study it is crucial to
recognise weaknesses and exploit advantages.
What kind of perfused heart
preparation can be used?
Although a number of other variants exist,
isolated perfused heart preparations are largely based on
adaptations of that originally described by Langendorff3 or
the more complex working preparation described by Neely4.
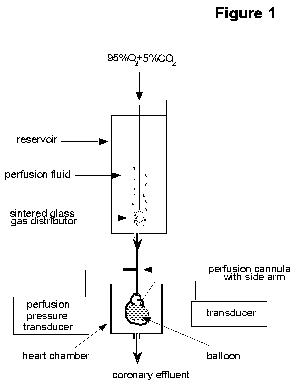 The Langendorff
heart preparation: As shown in Figure 1, this
involves the cannulation of the aorta which is then attached to a
reservoir containing oxygenated perfusion fluid. This fluid is
then delivered in a retrograde direction down the aorta either at
a constant flow rate (delivered by an infusion or roller pump) or
a constant hydrostatic pressure (usually in the range of
60-100mmHg). In both instances, the aortic valves are forced shut
and the perfusion fluid is directed into the coronary ostia
thereby perfusing the entire ventricular mass of the heart,
draining into the right atrium via the coronary sinus.
The Langendorff
heart preparation: As shown in Figure 1, this
involves the cannulation of the aorta which is then attached to a
reservoir containing oxygenated perfusion fluid. This fluid is
then delivered in a retrograde direction down the aorta either at
a constant flow rate (delivered by an infusion or roller pump) or
a constant hydrostatic pressure (usually in the range of
60-100mmHg). In both instances, the aortic valves are forced shut
and the perfusion fluid is directed into the coronary ostia
thereby perfusing the entire ventricular mass of the heart,
draining into the right atrium via the coronary sinus.
Constant flow or constant pressure perfusion?
Depending on the requirements of the experiment, both of the two
modes of perfusate delivery have advantages and disadvantages. In
both instances (in the absence of any imposed ischemia) and
depending upon species, the resulting coronary flow with a
blood-free perfusate is often in the range of 8-12 ml/minute/g
wet weight of tissue (a value which is several times that of
blood flow in vivo). Whilst constant flow perfusion adds an
additional element of constancy to an experiment it has the
disadvantage that, unlike constant pressure perfusion,
autoregulatory mechanisms are overridden and it does not
automatically alter the amount of perfusate delivered to the
whole heart when there are changes in heart rate or work or when
regional ischemia is imposed (under which circumstance the same
volume of perfusate may be forced through a much smaller
perfusion bed). Switching between constant flow and constant
pressure modes of perfusion is not straightforward and, with
simple apparatus, may not be feasible within a single
experimental protocol. Similarly, blood-perfused hearts where
circulating volumes are small (see later), may not easily lend
themselves to constant pressure perfusion. In order to address
this problem, Shattock et al5 developed an
electrical feedback system designed to control an isolated heart
perfused with a peristaltic pump. This system (now commercially
available through ADInstruments Ltd, Australia) allows the
perfuser to switch instantly between constant pressure and
constant flow modes of perfusion, enabling perfusion pressures
and coronary flow to be controlled over a wide range, and
providing a continuous on-line measurement of both perfusion
pressure and coronary flow if desired. This latter feature is of
considerable advantage in studies of vascular function. This
system has been used successfully with hearts from mice, rats,
guinea pigs and rabbits.
Using the rat as an example, the general
procedure for Langendorff perfusion is as follows:
Excision of the heart from the donor
animal: Isolation of the heart requires the donor to be
rendered unconscious prior to excision. Anesthesia can be induced
by inhalation of agents such as ether, halothane or
methoxyflurane or injection (via an intravenous or
intraperitoneal route) with agents such as pentobarbitone.
Intravenous administration to a conscious animal is usually via a
tail vein, whereas in the anesthetised state a femoral vein would
be the preferred route (the vein being accessed by a small skin
incision). An alternative to anesthesia is cervical dislocation
or concussion but in both instances there are major effects on
catecholamines and other circulating factors. However, there is
no correct or ideal procedure for rendering an animal
unconscious, nor is there an ideal anesthetic, each has its
advantages and disadvantages and these will vary from species to
species. Ether is hazardous as it is highly flammable and an
irritant to the animal and must only be used in a well ventilated
area. The most widely used anesthetic is pentobarbitone, but this
is a cardiovascular and respiratory depressant that can lead to a
reduction of cellular high energy phosphates6. Whatever the
choice of procedure (and this may be influenced by local animal
welfare regulations), every effort should be made to minimise
stress by keeping the animal in a quiet environment prior to
anesthesia and by minimising handling. Induction of anesthesia
should be as swift as possible, with the perception of pain
completely suppressed (this can be assessed by determining the
animal’s response to a stimulus such as the pedal withdrawal
reflex). Unless studying lipid or fatty acid metabolism (where
heparin has a lipolytic action) it is advisable to administer an
appropriate intravenous dose of heparin or another anticoagulant,
to prevent the formation of thrombi in the excised heart.
Once the animal is anesthetised the heart
can be excised. Generally, the diaphragm is accessed by a
transabdominal incision and cut carefully to expose the thoracic
cavity. The thorax is opened by a bilateral incision along the
lower margin of the last to first ribs, the thoracic cage is then
reflected over the animals head, exposing the heart. Some
investigators then cradle the heart between their fingers (it is
essential to do this gently to avoid contusion injury) and then
lift the heart slightly before incising the aorta, vena cava and
pulmonary vessels. Immediately after excision, hearts are usually
immersed in cold perfusion solution (4 degrees C to limit any
ischemic injury during the period between excision and the
restoration of vascular perfusion). Some investigators prefer to
cannulate the aorta in situ prior to excision of the heart, but
whichever the preferred procedure, it is important that vascular
perfusion be re-established as soon as possible after excision of
the heart. With practice and close proximity of the perfusion
apparatus and site of heart excision the entire process can be
accomplished in less than 30 seconds although some investigators
report times as long as 10 minutes (sufficient for unintentional
ischemic preconditioning or even stunning!)7.
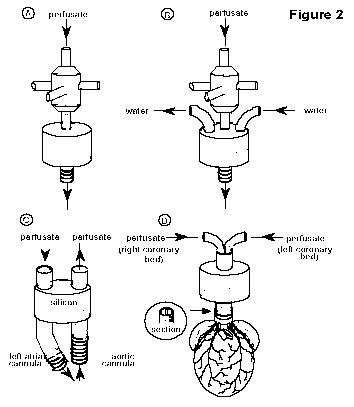 Cannulation and
re-establishment of vascular perfusion: The aortic perfusion
cannula (Figure 2) can be constructed from a variety of materials
including glass, plastic or thin walled stainless steel. The
external diameter is typically similar to, or slightly larger
than, that of the aorta (about 3mm for a heart from a 250g rat).
Several small circumferential grooves (Figure 2A) or a single flange is usually machined into the distal end of the
cannula to prevent the aorta from slipping off. Some cannulae (Figure 2B) are heated with water-jackets to prevent any
unwanted fall in perfusate temperature as it is delivered to the
heart (see later section on the importance of temperature
regulation).
Cannulation and
re-establishment of vascular perfusion: The aortic perfusion
cannula (Figure 2) can be constructed from a variety of materials
including glass, plastic or thin walled stainless steel. The
external diameter is typically similar to, or slightly larger
than, that of the aorta (about 3mm for a heart from a 250g rat).
Several small circumferential grooves (Figure 2A) or a single flange is usually machined into the distal end of the
cannula to prevent the aorta from slipping off. Some cannulae (Figure 2B) are heated with water-jackets to prevent any
unwanted fall in perfusate temperature as it is delivered to the
heart (see later section on the importance of temperature
regulation).
A water-jacketed reservoir, situated above the aortic cannula,
contains the perfusion fluid which is oxygenated via a sintered
glass gas distributor (for bicarbonate-based perfusion fluids
95%O2 + 5%CO2 is normally used). It is
advisable to have the perfusion fluid gently dripping from the
aortic cannula prior to cannulation since this helps minimise the
chance of air emboli at the time the heart is attached to the
cannula. Cannulation is aided by cutting along the aortic arch to
open it, thus giving a larger area for cannulation. Hearts should
be held gently between the tips of blunt-ended fine curved
forceps, taking care to avoid stretching or ripping of the aortic
wall. The aorta is then gently eased over the end of the cannula,
taking care not to insert the cannula too far into the aorta
since this would occlude the coronary ostia or damage the aortic
valve. The aorta
is then clamped to the cannula with a small blunt artery clip, whilst a ligature is rapidly tied around the
aorta, locking into the grooves; the artery clip can then
be removed. In the case of the
flanged cannula the aorta is slid down the cannula so that the
tie is against the flange. Full flow of perfusate should be
initiated as soon as the heart is mounted on the cannula.
Once the heart is securely attached to the
cannula any surplus tissue (such as bits of thymus, fat or lungs)
can be trimmed away. Drainage of coronary perfusate from the
right side of the heart via the pulmonary artery should be
unimpeded, however, in the course of cannulation it is possible
to accidentally ligate the pulmonary artery. Thus, to facilitate
adequate drainage it is advisable to make a small incision in the
base of the pulmonary artery using small pointed scissors. Some
investigators elect to cannulate the pulmonary artery,
particularly if they are interested in measuring A-V differences
in pO2 or are making a separate collection of cardiac
lymph. Some investigators also insert a small drainage catheter
in the apex of the left ventricle to prevent the accumulation of
any Thebesian flow in the left ventricular lumen. After its
passage through the coronary vasculature the coronary flow can
either be discarded or collected for analysis; if recirculating
perfusion is required the coronary effluent can be returned
(preferably via a 5µm filter) to the perfusion fluid reservoir
for reoxygenation.
Once cannulation is completed and coronary
perfusion initiated, contractile function and regular heart
rhythm will return within a few seconds but it may be 10 minutes
or more before maximum function is established. During this time,
various instrumentations of the heart can be undertaken. Most
studies require contractile function to be measured (not only for
the provision of baseline data but also to monitor the stability
of the heart and the extent of any disturbances of cardiac
rhythm). Although contractile activity can be assessed via a
strain gauge attached to the apex of the heart or an open tip
pressure transducer inserted into the left ventricle, the
preferred procedure involves the insertion of a compliant
intraventricular balloon. These balloons are often made from thin
silicone rubber or domestic food wrap. Not only is this ideal for
the measurement of isovolumic left ventricular function but it is
also a convenient means of measuring heart rate. For the balloon
insertion, the left atrial appendage is removed to provide a
clear field of view and a deflated balloon, attached to a short
rigid catheter and pressure transducer, is introduced into the
left ventricle via the mitral valve. The neck of the balloon is
then either sutured into position or firmly attached to the
cannula with a small piece of plasticine. Great care must be
taken while inserting and securing the balloon as it is very easy
to damage the heart - especially small hearts such as those from
mice or neonatal rats. The balloon is inflated with water from a
microsyringe until a left ventricular end diastolic pressure of
between 4-8 mm Hg is obtained (an excessively high balloon volume
should be avoided since it may cause tissue compression and
subendocardial ischemia which in turn will lead to an unstable
preparation with an increased propensity to arrhythmias). Once
the balloon is in position, left ventricular systolic, diastolic,
and developed pressure can be recorded. If the heart is to be
paced, one silver wire recording electrode can be hooked on the
ventricle with the reference electrode attached to the stainless
steel cannula. A bipolar stimulator is recommended in order to
avoid toxicity from electrolysis of the perfusion fluid which can
occur when unipolar stimulation is used. If an ECG is to be
recorded, the electrodes can be positioned as required, with
again, the stainless steel cannula making a suitable indifferent
electrode. Once instrumentation of the heart has been completed,
a temperature regulated heart chamber should be placed around the
heart and the top covered over with domestic food wrap or other
thin material.
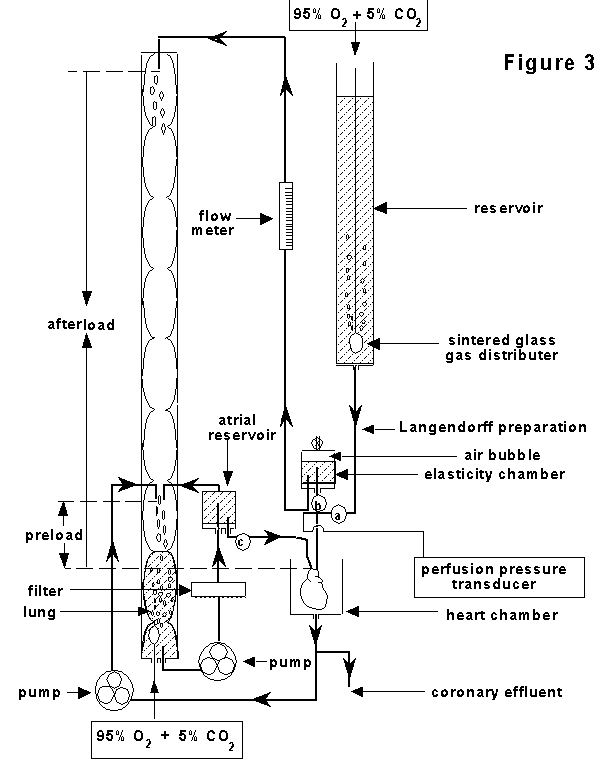
The working heart
preparation: As shown in Figure 3, this is a
more complex preparation with ventricular filling via the left
atrium and ejection in the normal direction via the aorta. This
preparation offers the advantage of an ability to measure pump
function with different filling pressures and afterloads. Rat
hearts are the most frequently used species for working heart
preparations but all species can be used - even the dog or pig.
Excision of the heart from the donor
animal: Since the first step in establishing a working heart
preparation is to set up a Langendorff preparation, the procedure
for anesthesia and excision is identical to that described
earlier.
Cannulation and re-establishment of
vascular perfusion: Again, the first steps are identical to
the Langendorff procedure described earlier, except that the
Langendorff perfusion line is usually attached to a side arm of
the aortic cannula (Figure
2C). Following the establishment of
perfusion (without the insertion of an
intraventricular balloon) there is just one additional step
(which proves most difficult to those learning the procedure)
namely the cannulation and secure tying off, without any leaks,
of the left atrium via one of the orifices of the pulmonary
veins. The dimensions and relative positions of the aortic and
atrial cannulae are critical to a successful preparation. Once
aortic and left atrial cannulation are accomplished, the
Langendorff perfusion line is clamped ("a" closed) and
perfusion initiated by unclamping the perfusion line from the
left atrium ("c" opened) whilst simultaneously
unclamping the aortic outflow line ("b" opened). In
this way, oxygenated perfusion fluid from a constant pressure
head left atrial perfusion reservoir (which is continuously
filled by a roller pump from a gassing reservoir which is
frequently referred to as ‘the lung’) flows under
gravity into the left atrial cannula. The preload of the
preparation is determined by the height of the overflow from the
atrial perfusion reservoir above the heart. This is usually set
to 20cm for isolated rat hearts but can be varied to suit other
preparations or to allow construction of "Starling
curves" relating preload to cardiac function. It is
important to stress that, in vivo, cardiac output is equal to the
venous return from the lungs to the left atrium - in the isolated
working heart the venous return is represented by the flow from
the left atrial cannula. An important point, often overlooked by
investigators when constructing a working heart apparatus, is
that the left atrial perfusion line must be capable of delivering
perfusion fluid at a rate sufficient to support the maximum
cardiac output of a working heart at any particular preload. If
the left atrial cannula is too small it will artificially limit
the cardiac output of the preparation. The problem is compounded
by the pulsatile nature of atrial filling, which means that the
atria only fill during about half of the cardiac cycle. To ensure
that this problem does not arise and that left ventricular
filling is not limited by inadequate left atrial inflow it is
essential to check that the left atrial perfusion line can
deliver a flow rate of at least twice the expected maximal
cardiac output. This is easily checked by running the apparatus
without a heart attached and measuring the flow from the left
atrial line - a rate of at least 150ml/min is recommended for a
1g heart. Having flowed from the left atrial cannula into the
left atrium, the perfusion fluid is ejected via the mitral valve
into the left ventricle from where it is ejected through the
aortic cannula against a hydrostatic pressure via the elasticity
chamber and flowmeter to the top of the lung. The afterload is
determined by the height of the column of fluid above the aortic
cannula. The elasticity chamber, which contains a known volume of
air for the working rat heart, mimics normal vascular elasticity.
It is an essential component of the perfusion circuit and without
it the heart will rapidly fail. In the course of left ventricular
ejection a portion of the perfusion fluid is forced into the
coronary ostia and thereby perfuses the coronary vessels of the
heart. The coronary effluent exits from the right heart into the
heart chamber from whence it may be sampled for assay or returned
(via a roller pump) to the lung for reoxygenation. Depending on
the species and experimental design, filling pressures are
usually in the range of 10-20 cmH2O and afterloads in
the range 60-100 cmH2O. Under these conditions and
using the heart from a 250g rat, coronary flows of up to 25
ml/minute and aortic flows of 50-80 ml/minute can be expected.
These can be measured by timed collection into measuring
cylinders or by in-line float or electromagnetic flow meters.
Summation of coronary and aortic flow gives the cardiac output.
Hearts may be paced or allowed to beat spontaneously under which
circumstances heart rate may be derived from a pressure recording
which is usually via a side arm of the aortic cannula. Because of
the large volumes of perfusion fluid pumped by the heart, the
working preparation usually operates in the recirculating mode
and for this reason it is essential to have an in-line filter
(5µm porosity) in the circuit to remove any particulate
contaminants which may originate from the heart, connecting
tubing, glassware or perfusion solutions.
What is the best perfusion
temperature?
Irrespective of whether a Langendorff or
working heart preparation is used it is obviously preferable to
perfuse at or near the normal body temperature of the species
under study. This value varies somewhat between species but in
general (unless as in some surgical studies hypothermia is
deliberately employed), most investigators elect to perfuse their
hearts at 37.0-37. 5°C. It cannot be stressed too strongly how
critical it is (and how difficult it is) to maintain good and
uniform temperature control, it is strongly recommended to have
permanent temperature sensing microthermisters at various parts
of the circuit. There are two basic approaches to maintaining the
heart, the perfusion circuit and the fluid it contains at the
correct temperature. The first is a thermostatically-regulated
cabinet in which moist warm air is circulated. These are rarely
used, they seldom work well (in part due to the loss of heat that
occurs every time the door is opened) and they can restrict
access to the heart. Most investigators choose to use a
thermostatically-controlled water-jacketed system in which all
glass reservoirs, the heart perfusion chamber and as many of the
delivery lines as possible are surrounded by rapidly flowing
water at 37.0-37. 5°C. To be effective, this requires a high
output, well regulated, water circulator and the careful design
of the circuit since some temperature drop across the apparatus
is inevitable. For this reason, key compartments such as the
heart chamber, the cannula assembly (if water-jacketed) and the
Langendorff or left atrial inflow lines should receive flow from
the circulator first. Bifurcation of the water lines should be
avoided as kinking of tubes may lead to different flow rates to
different parts of the apparatus. Finally, investigators should
avoid falling into the trap of setting the output of the
circulator at hyperthermic temperatures (e.g. 39°C) in an
attempt to compensate for any temperature drop between the
circulator and the heart chamber as this will inevitably cause
problems at some time or another. In general it is far better to
suffer a small degree of hypothermia in the circuit since
this will not damage the tissue and the relationship between
cardiac function and temperature, which, although very steep
below 35°C, is relatively flat between 35° and 37°C. A key
point is to avoid over-heating the tissue and to maintain a constant
temperature even if it is a little below that desired. Not
surprisingly, due to the necessarily very long perfusate delivery
lines, temperature control in hearts that are perfused in NMR
spectrometers are very vulnerable to poor temperature control.
Heat loss from the heart chamber can be reduced by the use of
cannulae attached to silicon (not red rubber) bungs the
diameter of which matches that of the heart chamber, thus
creating a tight seal. While this is easy to achieve in a working
heart preparation, it is harder in the Langendorff heart due to
the need for balloon insertion, although this can be overcome by
the creation of a small groove in the bung for the balloon
catheter. As mentioned earlier, an alternative approach is to
cover the area between a smaller bung and the heart chamber with
waxed laboratory film.
What indices of tissue
function, integrity and injury can be measured?
As already discussed, the isolated heart,
whether it be a Langendorff or working preparation, provides the
opportunity for the acquisition of a very wide spectrum of highly
reproducible data in a rapid and cost-effective manner. A variety
of physiographs can be used to record the data, computer based
recording devices offer some advantages over the more traditional
recorders in that they allow better data storage and analysis.
Morphology and vascular anatomy: The
perfused heart can readily be taken for examination by either
light or electron microscopy. In both instances it offers the
very important advantage that fixation can be by coronary
perfusion. For sequential studies, multiple microbiopsies can be
taken (starting at the apex of the heart and working towards the
base) at different times during a perfusion protocol (this
necessitates immersion fixation). In addition, whole hearts can
be used for gross morphology such as is required during infarct
size studies. Hearts can also be perfused with a variety of gels,
particles and resins that allow the specific visualisation of
vascular perfusion beds.
Biochemistry: There are a multitude
of measurements that can be made using the perfused heart.
Arterio-venous differences in substrates, metabolites such as
lactate, oxygen and a host of other markers of normal and
abnormal metabolism can be made. Leakage of cellular constituents
such as enzymes and proteins can readily be made for the
assessment of tissue injury and whole hearts or sequential
biopsies can be taken for metabolic analysis - typically of high
energy phosphates and the way in which they are influenced by
conditions such as ischemia and reperfusion. The ability to
perfuse hearts in an NMR spectrometer allows continuous on-line
measurement of metabolites and intracellular ions such as
calcium, protons or sodium. Transluminal or surface reflectance
fluorescence spectroscopy allows the continuous monitoring of
intermediates such as NADH or the contractile transients of ions
such as calcium. Suction microelectrodes allow continuous
measurement of interstitial potassium, calcium, pH or monophasic
action potentials. The perfused heart also provides an ideal
model for the delivery of vectors in gene transfer studies where
adenovirus or other vectors can be selectively delivered and
trapped in the coronary vasculature.
Cardiac rhythm and electrophysiology:
Electrocardiographic recordings allow the detection,
identification and quantification of abnormalities of cardiac
rhythm and microelectrodes allow further analysis - indeed, in
larger hearts such as the rabbit and the pig, conduction pathway
mapping and selective ablation is possible.
Cardiac contractile function:
Whereas the Langendorff preparation provides valuable information
on left ventricular systolic and diastolic pressures and their
derivatives, the working heart gives valuable data on cardiac
pump function. In addition, as mentioned earlier, various tension
recording devices may also be attached to the heart. Ultrasonic
crystals can be readily used for regional or transmural function
studies and various echo techniques can also be employed for
measurements of wall thickening. Pressure-volume relationships
can be studied with ease as can more rarefied indices of
contractile function such as Emax.8
Pharmacology: Both isolated heart
preparations are extremely valuable for assessing the direct
cardiovascular effects of various therapeutic agents in terms of
contractile function, electrical activity or metabolic function.
The option for recirculating and non-recirculating preparations
allows drug dose-response studies to be carried out with great
speed and reproducibility and with precise control over
concentration. Another advantage is to be able to rapidly washout
drugs from the circulation by replacing perfusion fluids.
Vascular biology: Whilst most
research in the isolated perfused heart has tended to focus on
the function and malfunction of the myocyte, using contractile
and metabolic endpoints, it should be stressed that both the
Langendorff and working heart can be used to study vascular
reactivity, endothelial and smooth muscle function and the effect
of a variety of interventions on coronary flow and its
distribution. Indeed, the isolated heart has been the cornerstone
of much of the work on the no reflow phenomenon.9
Should hearts be paced?
Like so many other facets of heart
perfusion, this decision is based on a compromise between
protocol requirements and quality of data. Left to contract at
its spontaneous rate, the isolated heart undergoes a small
progressive time-dependent decline in heart rate. Furthermore,
spontaneous heart rate in a perfused heart preparation is usually
significantly below the physiological norm. For rat hearts (which
have an in vivo rate of 350-400 beats/minute), heart rates of
250-320 beats/minute, can be expected. This changing
baseline will add additional variability to the data not only as
a consequence of the fall in heart rate plus
individual-to-individual variability in heart rate but also in
the value for left ventricular pressure which ( depending upon
whether the species under study has a positive or negative
staircase) will either increase or decrease as a consequence of
the fall in rate. Some investigators attempt to compensate for
this by expressing function in terms of pressure x rate product.
In vivo, the atria and sino-atrial node are
not perfused by coronary vessels but by extracardiac vessels,
which are severed when the heart is excised for perfusion. The
affected tissue is therefore dependent for its oxygen, nutrients
and temperature control upon either: (i) fortuitous superfusion
by coronary effluent flowing out of the right atrium or (ii)
deliberate superfusion from a cannula delivering warm perfusion
fluid. The implementation of an atrial superfusion line certainly
helps in maintaining temperature and a more stable heart rate.
However, it complicates the assessment of coronary flow. An
alternative approach is to immerse the entire heart in
oxygenated, temperature-regulated perfusion fluid.
Isolated hearts are easily paced to most
required levels and in studies where low heart rates are required
(and escape to a higher spontaneous rate may be threatened), the
sino-atrial node may be crushed to prevent natural impulse
generation. Pacing is not recommended in any studies of
arrhythmogenesis (where the pacing may modify the nature or
incidence of any arrhythmia) or the study of agents that
influence heart rate. During studies involving severe ischemia
many investigators choose to terminate pacing during the ischemic
interval and may delay restoration of pacing until several
minutes after the onset of reperfusion.
What is the maximum duration of
perfusion?
Perhaps the most frequent question posed by
lay visitors to a heart perfusion laboratory relates to the
longevity of the preparation. Clearly, from the moment an ex vivo
preparation is established, it will begin to deteriorate and the
rate will depend on a large number of factors including the skill
of the operator (avoiding contusion injury), the species, the
composition of the perfusion fluid, the presence or absence of
various drugs, age, heart rate and work load and the temperature
at which the studies are carried out. Certainly, investigators
should always undertake their own stability studies and establish
their own exclusion criteria. However, it is our experience that,
with both the Langendorff and working heart preparation, a
deterioration of contractile function (e.g. left ventricular
developed pressure or cardiac output) of 5-10%/hour can be
expected. However, this can be influenced greatly such that, in
some species, periods of hypothermic arrest with tissue
preservation for up to 24 hours or longer may be followed by a
return to approaching initial levels of function. The rate at
which a preparation deteriorates can be critical in the design
and interpretation of some studies where it may be necessary to
use time-matched controls with corrections for baseline
deterioration when comparing groups.
Should exclusion criteria be
used in perfused heart studies?
No self-respecting investigator who uses in
vivo preparations would design and undertake a protocol without
pre-defining criteria for the exclusion of preparations that are,
for one reason or another, unacceptable for use. Unfortunately,
only a minority of publications involving heart perfusion report
prospective exclusion criteria or policy for replacement of
excluded hearts (the latter being a potentially difficult issue
with the study of arrhythmias10). Those few publications that do cite exclusion
criteria often only state the lower limits of a functional index
(usually spontaneous heart rate or left ventricular developed
pressure), apparently accepting very high values however extreme.
The failure to apply rigorous prospective exclusion criteria in
heart perfusion studies probably has its origins in the
remarkable reproducibility of these preparations - however, this
is not an acceptable excuse for ignoring a fundamental
requirement of good protocol design.
What is the best perfusion
fluid composition?
The majority of studies in the literature
(with the exception of those involving NMR where phosphate often
has to be removed from the solution) are based on a bicarbonate
perfusion fluid as defined by Krebs and Henseleit.11
This perfusion fluid, which was supposed to mimic the key ionic
content of blood or plasma and have a pH of 7.4 at 37ºC, has the
following composition (in mM): NaCl 118.5, NaHCO3
25.0, KCl 4.7, MgSO4 1.2, KH2PO4
1.2, glucose 11.0 and CaCl2 2.5. Unfortunately, in
formulating this ‘physiological’ solution, Krebs and
Henseleit failed to take account of the fact that much of the
calcium in blood is bound to proteins and the realistic plasma ionised
calcium concentration is approximately half of the recommended
value of 2.5mM. As a consequence, for decades, heart perfusers
have used excessively high concentrations of calcium in their
perfusion fluid which usually do not contain protein or molecules
capable of binding calcium. This has meant that, in many studies,
hearts have been in a state of continuous inotropic challenge,
probably working near the upper limits of the calcium/function
curve. Nowadays, many investigators are rectifying this problem
by using ionised calcium concentrations which are in the range of
1.2-1.8mM.
In discussing calcium, it is important to
stress that, in preparing perfusion solutions containing both
calcium and phosphate ions, there is a risk of precipitation of
calcium phosphate particles which will occlude coronary arteries
and destroy a preparation. This is readily circumvented in
bicarbonate-buffered solutions by ensuring that the calcium
component is the last to be added and that the pH is lowered by
gassing with 95%O2 + 5%CO2 before adding
the calcium. Perfusion fluids should always be filtered (5µm
filter) before use to remove the remarkable number of particulate
impurities that can be present in even the purest commercial
chemical.
In discussing perfusion fluid composition,
it is important to consider the substrates that are provided to
support the large energy requirements of cardiac contractile
function. Traditionally, high concentrations (approximately 10mM)
of glucose are usually added as the sole substrate. The use of
‘diabetic’ levels of glucose reflects the fact that
normal physiological concentrations of glucose are unable to
sustain adequate function in the perfused heart in the absence of
insulin. Insulin can be added to overcome this problem, however,
it is rarely done - probably because of the other complex
cellular effects of insulin. The choice of glucose as the sole
substrate in most heart perfusion relies on the heart’s
ability to utilise almost any substrate as an efficient energy
source - this is despite the fact that, in vivo, fatty acids are
the predominant energy source. The general practice of avoiding
fatty acids as an energy source results primarily from the
difficulty of dissolving these agents in aqueous solutions and
the complication of frothing when the fatty acid-containing
solutions are gassed. The same reason, together with cost,
probably explains the general reluctance to add albumin, or other
oncotic agents, to perfusion solutions - a decision that
undoubtedly contributes to the severe edema which characterises
most perfused heart preparations. Drugs or other agents can be
added to any perfusion solutions; this is normally achieved by
adding them, in the desired concentration, to the basic perfusion
medium. However, in some cases, (e.g. unstable compounds)
concentrates can be infused via a side arm to the aortic or left
atrial cannula. In this instance, the concentration of the
infusate is set so as to achieve the desired circulating
concentration when the infusate is mixed with the perfusion fluid
as it enters the cannula.
What is the most common cause
of unstable or failing preparations?
Other than mishandling the heart during
excision, making an error in the formulation of a perfusion
solution, adding a toxic agent to a perfusion solution or the
occurrence of arrhythmias, the most common cause of contractile
failure in both working and Langendorff hearts is contamination -
either particulate or bacterial. The source of bacterial
contamination may be the perfusion apparatus itself where
inadequate washing at the end of an experiment or poor apparatus
design (small pockets between oversize tubing and a glass or
plastic connector which create spaces where the
substrate-containing perfusion fluid can be trapped) allows
bacteria to thrive. Alternatively, the perfusion solution may be
the cause with particulate impurities in reagents or bacterial
contamination that occurs during preparation or storage (it is
recommended not to store substrate-containing solutions for more
than a few hours). In both instances the problem can be
alleviated by: (i) filtration (5µm filter) in the course of
preparation and inclusion of filters in recirculating perfusion
circuits, (ii) making up fresh and not storing glucose- or
substrate-containing buffers which are good bacterial growth
media and (iii) thoroughly washing the perfusion apparatus with
either detergent or distilled water followed by (with appropriate
safety precautions) boiling water after every day of use. Some
investigators resort to the inclusion of broad spectrum
antibiotics in their perfusion media but this is not recommended.
If contamination of the perfusion apparatus does occur it may be
possible to remove it by washing with acid or detergent but
usually it is better to dismantle, wash and sterilise all
components. However, a well designed and properly washed
apparatus with well prepared perfusion solutions can be used for
years without contamination occurring.
Oxygen delivery: gassed
perfusion solution, perfluorochemicals, erythrocytes or blood?
Central to the survival of any perfused
organ is the continuous provision of oxygen in quantities
sufficient to support normal metabolism, the maintenance of
transmembrane ion gradients and, in the case of the heart, the
large amounts of energy needed to support contraction. In
conventional Langendorff and working heart preparations, oxygen
is provided by gassing the perfusion fluid with high
concentrations of oxygen, typically 95%O2 + 5%CO2
(the CO2 being required to achieve the correct pH in
bicarbonate-based buffers). If there are no fatty acid or protein
additions to the buffer then this is a straight forward procedure
with no complications from foaming. If foaming is a problem then
anti-foam agents can be added (but this is not recommended) or
membrane oxygenators may be employed.
Gassed asanguinous perfusion fluids have a
relatively low oxygen carrying capacity but a very high pO2
(> 500mmHg). The fact that they are able to provide sufficient
oxygen to the heart is testified by: (i) reducing the pO2
by using gassing mixtures containing only 70% oxygen does not
result in a loss of contractile performance, (ii) the venous pO2
is still relatively high indicating a reserve in oxygen
availability, (iii) perfusion fluid perfused preparations will
work for several hours without any great deterioration in
function and (iv) when challenged with inotropic agents perfusion
fluid perfused hearts can increase their work for sustained
periods without injury. However, it should be stressed that
asanguinous perfusion fluids can only provide sufficient oxygen
if the coronary flow rates are several times the physiological
norm.
Driven by concerns over the transmission of
infections during blood transfusion, there has been considerable
research into developing blood substitutes and especially
perfluorochemical oxygen-carrying hemoglobin substitutes. These
agents, when used as emulsions with aqueous media, are able to
deliver more than twice as much oxygen.12 As a
consequence perfluorochemicals have been used by some
investigators in perfusion solutions but this practice has not
been widely adopted. This probably reflects the general
satisfaction that oxygenated aqueous media are able to deliver
sufficient oxygen to most isolated heart preparations. As a
consequence, most work in the perfluorochemical area has resolved
around using these agents to provide oxygen to tissue which is
deprived of adequate flow. In this connection there are many
studies of these agents as additives to cardioplegic solutions or
as agents to limit the evolution of myocardial infarction.
However, again, they have not been introduced into widespread
use.
The artificially high coronary flow rates
and high pO2 of asanguinous perfusion solutions,
together with the absence of the many key components of blood,
makes the concept of perfusing isolated hearts with blood an
attractive option. Certainly, blood perfusion is used
successfully with many other organs; it would be expected to
allow near normal coronary flow rates; its protein content should
help alleviate the unwanted edema that characterises perfusion
fluid-perfused hearts and it should also allow the heart to be
exposed to blood-borne elements such as neutrophils. The
widespread use of blood in heart perfusion has probably been
discouraged by: (i) the volume of blood needed (perfusing one rat
heart would require the blood of several rats), (ii) the
inability to use donor blood from some (but not all) larger
species because they have larger erythrocytes than the rat and
cannot traverse rat heart capillaries and (iii) the difficulty in
oxygenating the blood since conventional gassing creates
extensive foaming and damage to blood cells. However, all of
these problems can be circumvented either through the use of
parabiotic preparations with support rats or the use of membrane
oxygenators. As a consequence, a number of blood or erythrocyte
preparations have been developed13,14,15,16 and
used with great success.
Blood perfusion: The first report of
an attempt to develop an isolated blood perfused rat heart was
published by Gamble et al in 1970.14 A
ventilated support rat was placed in a chamber containing
humidified air at 37°C and the left carotid artery and right
jugular vein were cannulated for the supply and return of blood
to an isolated Langendorff heart. Blood from the carotid artery
of the support rat first flowed into a latex bag, the volume of
which was maintained constant by a feedback device. The blood was
then pumped to the aortic cannula of the isolated heart and
returned to the jugular vein of the support rat. In 1988, Abraham
et al15 modified the preparation by removing the pump.
Blood perfusion with a support animal
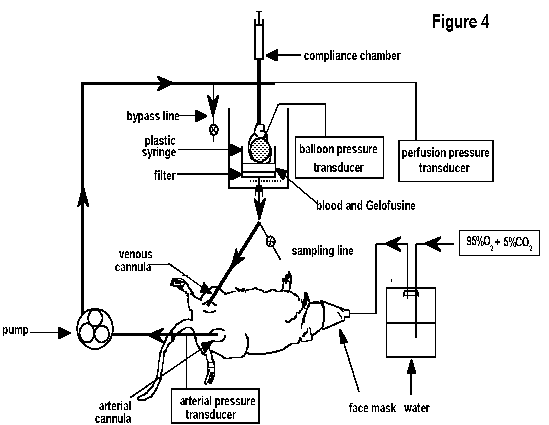
for oxygenation: The preparation currently used in our
laboratory for rats and rabbits, is based on the procedure
described by Qiu et al13,16, which is a combination of the two methods
mentioned above. The details for establishing this preparation (Figure 4) in the rat are outlined below.
Support rats (350-400g) are anesthetised
with sodium pentobarbitone (60mg/kg, ip) and anticoagulated with
heparin (1000 IU/kg). The left femoral artery and right femoral
vein are exposed by blunt dissection and cannulated for the
eventual supply and return of blood to an isolated Langendorff
perfused heart from another animal of the same species. An
extracorporeal circuit between these two vessels, primed with a
Gelofusine solution (a plasma substitute consisting of gelatin,
sodium chloride and water) is constructed and the flow of blood
through this is gradually established and maintained for a short
time (usually 20 minutes) so as to ensure that the priming
solution is adequately mixed with the blood of the support animal
and the entire preparation is stable. It is advantageous to
decrease the hematocrit of the perfusing blood to approximately
28-30% by mixing it with Gelofusine. This decrease in the
viscosity allows for better flow and also helps minimise the
possibility of clotting. The gradual establishment of the
extracorporeal circuit is achieved via an in-line peristaltic
pump which is positioned on the arterial outflow of the support
animal and flow through this circuit is gradually increased over
10 minutes to its final required value (which depends on the
species used and the size of the heart but for a 1g rat heart the
flow rate would normally be in the range 2-3 ml/minute). The
gradual increase in flow through the extracorporeal circuit is
necessary to prevent the sudden drop in arterial pressure that
would have occurred if the final flow rate of had been
established abruptly. The feedback system described earlier can
be used with this preparation to perfuse the isolated heart
either under conditions of constant flow or constant pressure.
The blood is pumped through a cannula (to which the aorta of the
perfused heart will subsequently be attached) and returned, by
gravity, via a reservoir (comprising of the bottom half of a
large syringe positioned in a water-jacketed heart chamber) and
filter (200µm filter) to the venous inflow line of the support
animal. Meanwhile, the isolated rat heart is harvested and
prepared exactly as described in earlier sections. The heart can
also be instrumented with an intraventricular balloon and other
devices exactly as described earlier. The extracorporeal circuit
then flows from the left femoral arterial supply line of the
support animal to the isolated heart. The coronary effluent from
the perfused heart is then returned, by gravity, via a reservoir
and filter to the venous inflow line of the support animal.
During periods of global ischemia, the blood can be diverted from
the isolated heart by opening the bypass line (which has been
positioned to flow into the reservoir) and clamping the line to
the heart.
It is possible to artificially ventilate
the support rat, however, it is usually sufficient to allow the
animal to spontaneously breathe a mixture of 95% O2 +
5% CO2 through a Venturi face mask, the flow rate of
which is adjusted to maintain blood pO2 and pCO2
within the physiological range. Before placing the face mask over
the support animal, the tongue should be gently pulled slightly
out of the mouth and placed to one side to ensure unobstructed
breathing. Body temperature, monitored by a rectal thermometer,
should be stabilised by means of a thermostatically-controlled
heating pad. Blood pressure should be monitored by a pressure
transducer attached to the arterial line. Blood gas and
electrolyte levels of the support rat should also be checked
constantly and corrected when necessary. During the course of the
experiment, donor blood from another rat can be transfused as
required to maintain the volume and stability of the preparation.
Additional anticoagulant and anesthetic can be administered as
required.
The above method of blood perfusion
requires that very careful attention be paid to the hemodynamics
and blood chemistry of the support animal, with constant
monitoring of vital signs, breathing and the colour of mucous
membranes. An air-filled syringe (Figure 4) above
the perfusion cannula acts as a compliance chamber, which serves
to dampen oscillations in perfusion pressure which occur as a
consequence of the contraction of the isolated heart and the
peristaltic action of the pump.
Advantages and disadvantages of the
preparation: There are a number of major advantages with this
preparation. Firstly, in our experience, it is even more stable
than the isolated perfusion fluid perfused heart, with left
ventricular developed pressure deteriorating less than 5%/hour.
The blood perfused heart suffers far less edema and excellent
pressure development is observed (in the range 130-180 mmHg when
end diastolic pressure is in the range 4-8 mmHg). Coronary flow
rate (2-3 ml/minute) is much closer to the physiological range
than that with perfusion fluid perfused preparations and the
preparation allows one to study the effect of blood elements such
as neutrophils on cardiac function. By using two support animals
attached to one heart it is possible to study the effect of
transient exposure (or removal) of factors such as neutrophils.
The preparation can be used to study global or regional ischemia
at zero or reduced flow rates exactly as a perfusion
fluid-perfused heart and is amenable to the same wide range of
biochemical, morphological, pharmacological and functional
assessments. With careful attention to the support animal, it is
our experience that one support rat can be used for several hours
and allow the sequential study of more than one isolated heart.
A potential disadvantages of the support
animal method of blood perfusion is that any substance released
by, or administered to, the isolated heart will normally be
transmitted to the support animal with possible deleterious
consequences. On the other hand, in some instances, the support
animal may exert a beneficial action by metabolising or excreting
such substances. Another related disadvantage of this preparation
is that any deterioration of the support animal will threaten the
survival of the isolated heart. However, with care, a replacement
support animal can be introduced into the perfusion circuit. When
perfusing with either whole blood or a washed red cell
preparation, care must be taken to ensure that the perfusate does
not come into contact with glass as this will promote red cell
hemolysis.
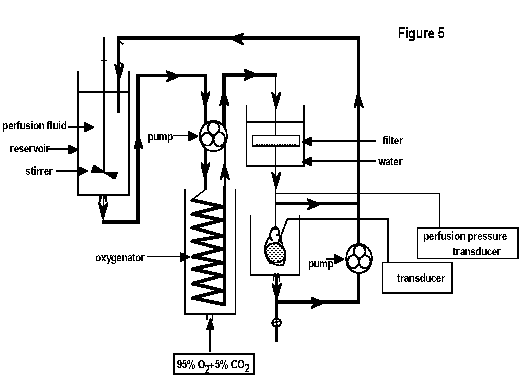
Erythrocyte perfusion:
If the use of a support animal is not feasible then an
alternative approach is to undertake isolated heart perfusion
with washed red blood cells using a simple membrane oxygenator
constructed from coils of thin walled silicone rubber tubing. In
1979 Bergmann et al17 reported a preparation in which isolated
rabbit hearts were perfused with perfusion fluid containing red
cells at a hematocrit of 25% or 40%. A key feature of this
preparation was the use of blood cells from a different species
(sheep) which are small enough to traverse the capillaries of the
rabbit heart. They found that, in addition to exhibiting an
enhanced stability when compared to crystalloid perfused hearts,
the red cell perfused hearts were less likely to develop
significant edema. The model currently used in our laboratory18
is essentially similar (Figure 5). A red cell
(bovine blood) suspension is kept in a reservoir which is stirred
continuously to prevent red cell sedimentation. From this
reservoir the red cell suspension is pumped in an artificial
‘lung’, comprising of gas permeable silicon tubing
wound into a coil. This tubing is housed in a water-jacketed
chamber, which maintains the perfusate at 37ºC. We find it to be
advantageous to pump the red cell perfusate both into and out of
the oxygenator since pumping on one side of the circuit can lead
to stretching and distortion of the oxygenator tubing. However,
it is essential that the pump speeds are identical to prevent
emptying or pooling of blood in the oxygenator. In addition, 95%O2
+ 5%CO2 is pumped into the reservoir so as to maintain
oxygenation of the red cells, whilst keeping pH within the
physiological range. The perfusate is then passed through a white
cell filter, which has two functions: (i) the large surface area
of the filter and its immersion in heated water provides an
efficient method of temperature regulation and (ii) the filter
protects the heart against micro-emboli.
Excision of the heart from the donor
animal: The procedure for anesthesia and excision is
identical to that described in the section on blood perfusion
with a support animal.
Preparation of a red cell perfusate:
Preparation of a red cell perfusate is more time-consuming than
the preparation of a simple perfusion solution. Fresh blood (most
commonly ovine or bovine) is collected into vessels containing
5,000 IU heparin/litre of blood and 0.2 MU benzylpenicillin
/litre of blood. After filtering (200µm filter) the blood is
centrifuged at 1000g for 20 minutes, after which the supernatant
and white cell layer are removed and discarded and the red cells
resuspended in nominally calcium-free perfusion fluid. This
process is repeated until the supernatant is clear. If the cells
are to be used the next day, it is advisable to omit the final
resuspension, instead storing the packed cells in a refrigerator
(4ºC) and washing twice on the day of use. Following the final
wash, the red cell concentrate is mixed 1:1 with a
dextran/albumin solution, made by adding 250ml of deionised water
to 500 ml of sterile Gentran 70 ® (a plasma expander consisting
of 6% dextran and 0.9% sodium chloride). Electrolytes and glucose
are added in the following concentrations (mM): NaCl 118.5, KCl
3.8, KH2PO4 1.2, NaHCO3 25.0,
MgSO4 1.0 and glucose 10.0. After filtration (5µm
porosity) the solution is warmed and 3g of albumin (Fraction V,
Sigma) added. Prior to perfusing, the ionic composition of the
solution should be checked using a blood gas/electrolyte analyser
and corrected if necessary (e.g., the final calcium concentration
should approximate the ionised calcium concentration in blood).
Dextran is used to prevent microagglutionation and gentamicin (2
mg/litre) can be added to retard bacterial growth.
Advantages and disadvantages of the
preparation: The advantages with this preparation are similar
to those for the blood perfused preparation which uses a support
animal: (i) it is more stable than the isolated perfusion fluid
perfused heart, with pressure development remaining stable for
extended periods of perfusion (approximately 5 % loss/hour), (ii)
the red cell perfused heart suffers far less edema than the
perfusion fluid perfused heart, and (iii) coronary flow rate (2-3
ml/minute) is much closer to the physiological range than that
with the perfusion fluid perfused preparation. Pressure
development in the erythrocyte perfusion preparation is usually
not as great as that seen in the blood perfused heart and is
similar to that seen in the buffer perfused heart.
Perfusing hearts with blood from another
species raises the possibility of an adverse immunological
reaction, but as the blood is normally depleted of white cells
and plasma proteins during its preparation, significant reactions
are unlikely. However, progressive hemolysis of the red cells
during use is unavoidable, care should be taken to avoid contact
with glass (use plastic only) during the preparation or usage of
the blood, as this will greatly accelerate red cell haemolysis.
How can ischemia be best
induced?
Whether perfused with an asanguinous
solution, washed red cells or blood in the Langendorff or the
working mode, many investigators use the isolated heart for the
study of regional or global ischemia. The isolated preparation is
ideally suited to such studies since pump failure or lethal
arrhythmias do not necessarily terminate an experiment as would
be the case with in vivo studies. Global (whole heart) zero-flow
ischemia is readily induced in the Langendorff and the working
heart simply by occluding the perfusion inflow lines. Graded
whole heart ischemia at various degrees of flow can also be
readily induced in the Langendorff preparation but is difficult
to achieve in the working heart (as a consequence, investigators
often switch the working heart temporarily back to the
Langendorff mode for such treatment). Regional ischemia can also
be induced in both preparations by ligating a coronary artery
(usually the left main) and the size of the ischemic zone can be
influenced by the positioning of the occlusion point. To prevent
tearing of tissue and also to facilitate reperfusion, the
occluding ligature is usually tied against a small length of
plastic tubing. Reperfusion can be achieved by cutting the
ligature. The relatively uniform coronary artery anatomy of the
rat heart offers a great advantage in that ischemic zones of very
similar sizes are usually created. The size of the ischemic zone
can be easily estimated by the percent fall in coronary flow (in
constant pressure perfusions) or increase in coronary vascular
resistance (in constant flow preparations). Validation of
occlusion is often made by transient infusion of a coloured dye
or fluorescent particles. In the Langendorff heart a novel dual
lumen perfusion cannula19 (Figure
2D) can be used to facilitate
independent perfusion of the left and right coronary ostia. This
cannula is a powerful tool for investigating the regional effects
of drugs - it also offers the unique opportunity of creating
regional low flow ischemia in the rat heart. Isolated hearts also
provide the opportunity for studying hypoxia and reoxygenation,
again either regional or global.
Concluding comments.
Having selected the isolated perfused heart
as a model to investigate a cardiovascular phenomenon, the choice
of preparation is very wide. Blood perfused or perfusion solution
perfused, Langendorff or working, each has its own advantages and
disadvantages, both of which can be established in the quest for
a better understanding of cardiac function and malfunction.
Although the investigative power of the preparation is great, as
with all experimental models, they are fraught with potential
pitfalls, which must be recognised and addressed. This article
has attempted to address many of these issues in the hope that it
will assist investigators to make the best possible use of this
experimental model.
References
1. Hearse DJ and Sutherland FJ.
Experimental models for the study of cardiovascular function and
disease. Pharmacological Research 2000 (in press).
2. Galinanes M and Hearse DJ. Species
differences in susceptibility to ischemic injury and
responsiveness to myocardial protection. Cardioscience 1990;1:
127-143.
3. Langendorff O. Untersuchungen am
uberlebenden Saugethierherzen. Pflugers Archives fur die Gesamte
Physiologie des Menschen and der Tiere 1895;61:291-332.
4. Neely JR, Liebermeister H, Battersby EJ
and Morgan HE. Effect of pressure development on oxygen
consumption by isolated rat heart. American Journal of Physiology
1967; 212:H804-H814.
5. Shattock MJ, Miller JIA, Bray DG and
Waldron CB. An electronic feed-back circuit to control a
peristaltic pump for constant-pressure perfusion of isolated
hearts or other organs. Journal of Physiology 1997; 505:4P.
6. Bretschneider HJ, Hubner G, Knoll D,
Lohr B, Nordbeck H and Spieckermann PG. Myocardial resistance and
tolerance to ischemia: physiological and biochemical basis.
Journal of Cardiovascular Surgery 1975; 16 ,3:241-260.
7. Awan MM, Taunyane C, Aitchison KA,
Yellon DM and Yellon DM. Normothermic transfer times up to 3 min
will not precondition the isolated rat heart. Journal of
Molecular and Cellular Cardiology 1999; 31:503 - 511.
8. Fukunami M and Hearse DJ. The inotropic
consequences of cooling:studies in the isolated rat heart. Heart
and Vessels 1989; 5:1-9.
9. Kloner RA and Alker KJ. The effect of
streptokinase in intramyocardial hemorrage, infarct size, and the
no-reflow phenomenon during coronary reperfusion. Circulation
1984; 3:513-521.
10. Curtis MJ. Models for the study of
arrhythmias in myocardial ischaemia and infarction: the use of
the rat. Journal of Molecular and Cellular Cardiology 1987;
19:399-419.
11. Krebs HA and Henseleit K.
Untersuchungen ueber die Harnstoffbildung im Tierkoerper.
Hoppe-Seyler’s Zeitschrift fur Physiologische Chemie 1932;
210:33-36.
12. Lowe KC. Perfluorinated blood
substitutes and artificial oxygen carriers. Blood Reviews 1999;
13:171-184
13. Qiu Y and Hearse DJ. Comparison of
ischemic vunerability and responsiveness to cardioplegic
protection in crystalloid-perfused versus blood-perfused hearts.
Journal of Thoracic and Cardiovascular Surgery 1992; 103:960-968.
14. Gamble WJ, Conn PA, Kumar AE, Plenge R
and Monroe RG. Myocardial oxygen consumption of blood-perfused,
isolated, supported, rat heart. American Journal of Physiology
1970; 219:H604-H612.
15. Abraham RGM, Merserau WA and Chiu RC-J.
A simplified alloperfused rat heart model for studying myocardial
protection. Journal of Investigative Surgery 1988:107-116.
16. Qiu Y, Manche A and Hearse DJ.
Contractile and vascular consequences of blood versus crystalloid
cardioplegia in the isolated blood-perfused rat heart. European
Journal of Cardio-thoracic Surgery 1993; 7:137-145.
17. Bergmann SR, Clarck RE and Sobel BE. An
improved isolated heart preparation for external assessment of
myocardial metabolism. American Journal of Physiology 1979;
236:H644-H651.
18. Connaughton M. Aspects of ischaemia and
reperfusion in the isolated rat heart. A thesis presented for the
University of London in fulfilment of the regulations for the
degree of Doctor of Medicine 1997.
19. Avkiran M and Curtis MJ. Independent
dual perfusion of left and right coronary arteries in isolated
rat hearts. American Journal of Physiology 1991; 261:H2082-H2090.
Return to the HELP Table of Contents
 The Langendorff
heart preparation: As shown in Figure 1, this
involves the cannulation of the aorta which is then attached to a
reservoir containing oxygenated perfusion fluid. This fluid is
then delivered in a retrograde direction down the aorta either at
a constant flow rate (delivered by an infusion or roller pump) or
a constant hydrostatic pressure (usually in the range of
60-100mmHg). In both instances, the aortic valves are forced shut
and the perfusion fluid is directed into the coronary ostia
thereby perfusing the entire ventricular mass of the heart,
draining into the right atrium via the coronary sinus.
The Langendorff
heart preparation: As shown in Figure 1, this
involves the cannulation of the aorta which is then attached to a
reservoir containing oxygenated perfusion fluid. This fluid is
then delivered in a retrograde direction down the aorta either at
a constant flow rate (delivered by an infusion or roller pump) or
a constant hydrostatic pressure (usually in the range of
60-100mmHg). In both instances, the aortic valves are forced shut
and the perfusion fluid is directed into the coronary ostia
thereby perfusing the entire ventricular mass of the heart,
draining into the right atrium via the coronary sinus.


