At the University of South Alabama, the College of Engineering is ready to help you prepare for a successful career. Our city and surrounding region are home to many engineering and technology companies that encourage and support our students with internships, co-ops, and excellent career opportunities.
Engineering Jag Buddy Program! - Sign Up Today!
Find Your Path in Engineering
|
Latest News
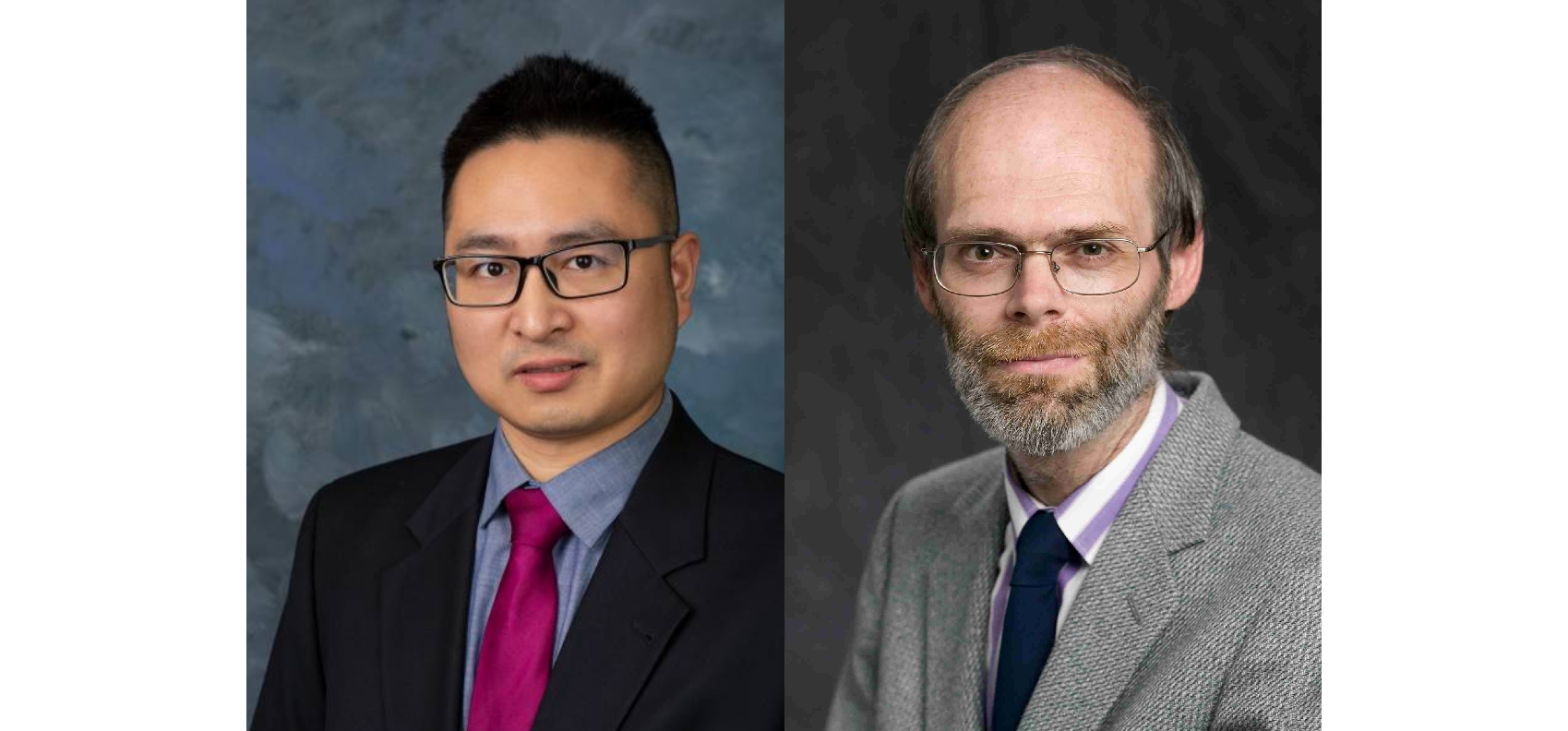
Dr. Woods and Dr. Wu honored by MACE awards
Tuesday - March 4, 2025
Launching a Career
Friday - August 16, 2024
USA Engineering Professor Receives New NSF Grant for Novel AI Computing Systems
Tuesday - August 13, 2024
South Launches New Faculty Ambassador Program
Tuesday - July 30, 2024