DEA Registration
Introduction
DEA Registration Quick Guide
Each PI who uses or receives DEA-controlled substances must obtain an individual DEA Research Registration (Form 225).
PIs are individually responsible for:
- Ordering controlled substances
- Maintaining accurate usage logs
- Conducting and documenting inventory
Biological Resources can provide support.
We can:
- Assist with the DEA registration process
- Help establish compliant record-keeping procedures
- Assist with controlled substance ordering, as needed
Original Application - To obtain a new DEA license for research purposes, log on to the DEA website and click on “New Applications.”
Once redirected, see the pages below for step-by-step instructions for completing this online application. The application takes about 15 minutes to complete, but it can take 6-8 weeks to obtain the license so plan ahead. If you require additional assistance during the application process, you may contact the IACUC/IBC Administrator or Biological Resources.
Application Renewal – The DEA license needs to be renewed annually. Use the same DEA website address, but select “Renewal Applications” instead.
Once registered, send a copy of your certificate to:
- Leigh Ann Wiggins, Senior Manager, Biological Resources – for ordering and transfer documentation
- Danielle Miller, IACUC/IBC Administrator – for IACUC protocol congruency tracking
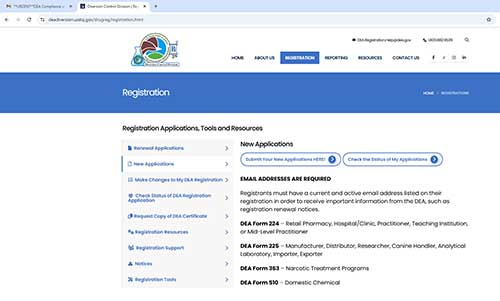
NEW APPLICATIONS
Select “new application” on the DEA website:
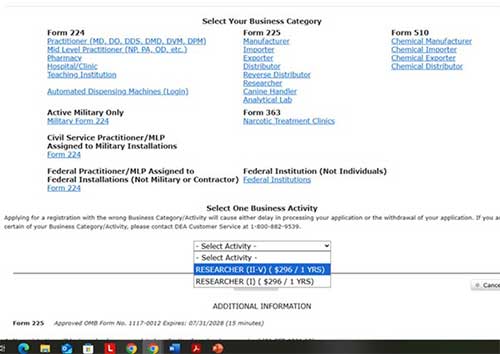
Select “researcher” under “Form 225” and “RESEARCHER II-V” from the drop-down:
Leave the next page blank unless you wish to designate a POA. Click “proceed”.
Complete the personal information section. This must match where the controlled substances will be STORED. You can have your registration mailed to your department by completing the mailing address for your department.
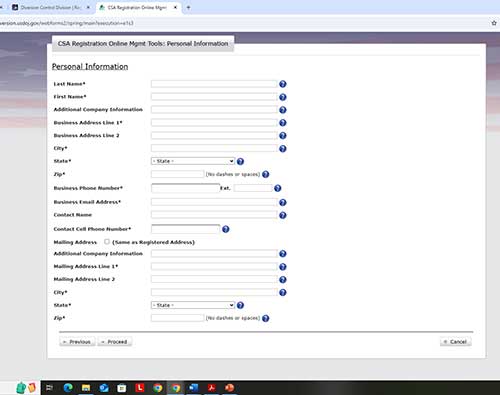
Follow the prompts to validate your email address. Click proceed.
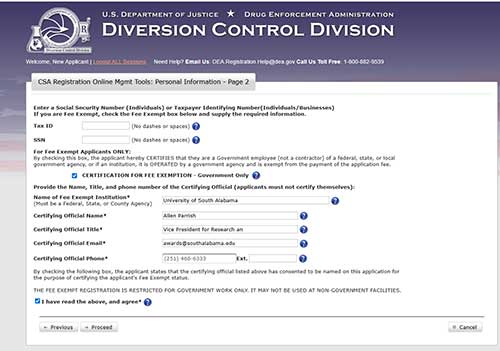
On the next page, enter your SSN and check the fee exemption box.
The University of South Alabama (USA) is a state institution and is fee exempt for DEA registration. When completing the application, the researcher should list either the Chair of their department or Dr. Allen Parrish, Vice President for Research and Economic Development, as the certifying official. For Dr. Parrish, they should use the following contact email: awards@southalabama.edu. Use the following contact phone number: 251-460-6333.
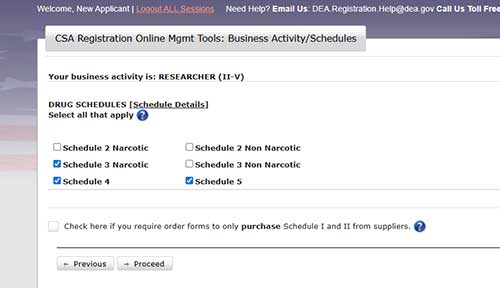
Select the drug schedules required for your research. (Ethiqa XR and buprenorphine are narcotic schedule III drugs. Ketamine and Euthasol are non-narcotic schedule III drugs.) Click proceed.
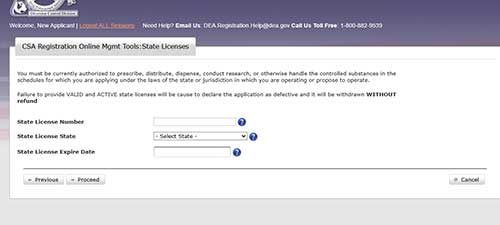
Do NOT complete this section. Alabama does not issue CSRs to researchers. Click proceed.
Answer the following questions and click proceed.
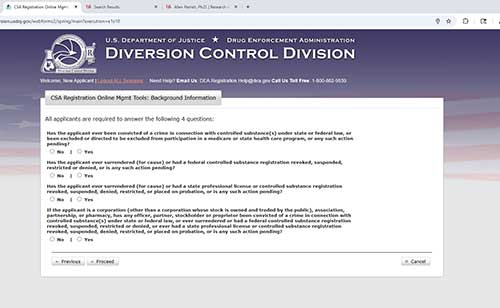
Click proceed again to skip the next page.

On the final page:
Check that all information is correct. Remember, the registered address is the PHYSICAL LOCATION of your substance storage.
If everything is correct, e-sign the application and follow the prompts to submit the application.
Be sure to print the confirmation page of your submission.
You should receive an email from the DEA with the following in the subject line: “DEA Registration Application Receipt”.
The DEA license needs to be renewed annually. Use the same DEA website address, but select “Renewal Applications” instead. Follow the prompts, using the same information as was used for your prior (new or renewal) application, making changes where appropriate (e.g., change of address, etc.)